Acute lymphoblastic leukemia (ALL) is a cancer of the lymphoid lineage of blood cells, characterized by the overproduction of immature lymphocytes, called lymphoblasts, and their accumulation in the bone marrow. The accumulation of lymphoblasts in the bone marrow interferes with the production of red and white blood cells and platelets, leading to the symptoms of leukemia. These symptoms can include the fatigue and pallor of anemia, increased risk of infection, and easy bleeding and bruising. In addition, the physical crowding of lymphoblasts within the confined space of the bone marrow can lead to arm or leg pain.
ALL is most common in childhood, with a peak incidence at 2-5 years of age and another peak in old age. About 6,000 cases are reported in the United States every year. Internationally, ALL is more common in Caucasians than in Africans; it is more common in Hispanics and in Latin America. Cure is a realistic goal and is achieved in more than 80% of affected children, although only 20-40% of adults are cured. "Acute" is defined by the World Health organization standards, in which greater than 20% of the cells in the bone marrow are blasts. Chronic lymphocytic leukemia is defined as having less than 20% blasts in the bone marrow.

Maps, Directions, and Place Reviews
Signs and symptoms
Initial symptoms on presentation can be nonspecific, particularly in children. In a meta-analysis, over 50% of children with leukemia had one or more of these five features on clinical presentation: palpable liver (64%), palpable spleen (61%), pallor (54%), fever (53%), and bruising (52%). Additionally, recurrent infections, fatigue, limb pain, and lymphadenopathy can be prominent features of leukemia. These symptoms largely reflect the normal and healthy blood cells crowded out by malignant and immature white blood cells (WBC) in the bone marrow. The resulting bone marrow failure causes dysfunctional erythrocytes, leukocytes, and platelets. The B symptoms, such as fever, night sweats, and weight loss, are often present as well. While WBC count at initial presentation can vary, circulating lymphoblasts are generally seen on peripheral smears. Central nervous system (CNS) symptoms extending from cranial neuropathies to meningeal infiltration can be identified in <10% of adults and <5% of children with ALL, particularly mature B-cell ALL (Burkitt leukemia) at presentation.
While many presenting symptoms can be found in self-limited childhood illness, persistent or unexplained common signs should be worked up for malignancy. Laboratory tests that might show abnormalities include blood count tests, renal function tests, electrolyte tests, and liver enzyme tests.
The signs and symptoms of ALL are variable but follow from bone marrow replacement and/or organ infiltration. Below include
- Generalized weakness and fatigue
- Anemia
- Dizziness
- Headache, vomiting, lethargy, nuchal rigidity, or cranial nerve palsies (CNS involvement)
- Frequent or unexplained fever and infection
- Weight loss and/or loss of appetite
- Excessive and unexplained bruising
- Bone pain, joint pain (caused by the spread of "blast" cells to the surface of the bone or into the joint from the marrow cavity)
- Breathlessness
- Enlarged lymph nodes, liver and/or spleen
- Pitting edema (swelling) in the lower limbs and/or abdomen
- Petechiae, which are tiny red spots or lines in the skin due to low platelet levels
- Testicular enlargement
- Mediastinal mass
Acute Lymphoblastic Leukemia Type B Survival Rate Video
Cause
Functional germline mutations of some cancer-related genes have been found in familial ALL or enriched in radic cases (e.g., PAX5, ETV6 and CDKN2A), accounting for ALL susceptibility for a small proportion of people, while large genome wide association studies revealed multiple inherited predisposition to ALL risk, including single nucleotide polymorphisms (SNPs) at ARID5B, IKZF1, CEBPE, PIP4K2A, GATA3, and CDKN2A loci among diverse populations.
Epidemiological studies suggest that environmental factors on their own make only a minor contribution to disease risk, but environmental factors may interact with genetics. Genome-wide association studies have found associations with a number of genetic single-nucleotide polymorphisms, including ARID5B, IKZF1 and CEBPE.
There is an increased incidence in people with Down syndrome, Fanconi anemia, Bloom syndrome, X-linked agammaglobulinemia, severe combined immunodeficiency, Shwachman-Diamond syndrome, Kostmann syndrome, Neurofibromatosis type 1, Ataxia-telangiectasia, Paroxysmal nocturnal hemoglobinuria, and Li-Fraumeni syndrome. There is an increased risk in people with a family history of autoimmune diseases, particularly autoimmune thyroid diseases (namely Graves' disease or Hashimoto's thyroiditis).

Pathophysiology
In general, cancer is caused by damage to DNA that leads to uncontrolled cellular growth and spreads throughout the body, either by increasing chemical signals that cause growth or by interrupting chemical signals that control growth. Damage can be caused through the formation of fusion genes, as well as the dysregulation of a proto-oncogene via juxtaposition of it to the promoter of another gene, e.g. the T-cell receptor gene. This damage may be caused by environmental factors such as chemicals, drugs or radiation, and occurs naturally during mitosis or other normal processes (although cells have numerous mechanisms of DNA repair that help to reduce this).
ALL is associated with exposure to radiation and chemicals in animals and humans. High level radiation exposure is a known risk factor for developing leukemia, as found by studies of survivors of atom bomb exposure in Hiroshima and Nagasaki. In animals, exposure to benzene and other chemicals can cause leukemia. Epidemiological studies have associated leukemia with workplace exposure to chemicals, but these studies are not as conclusive. Some evidence suggests that secondary leukemia can develop in individuals treated for other cancers with radiation and chemotherapy as a result of that treatment.

Diagnosis
Diagnosing ALL begins with a medical history, physical examination, complete blood count, and blood smears. Because the symptoms are so general, many other diseases with similar symptoms must be excluded. Typically, the higher the white blood cell count, especially if the number of cancerous malignant abnormal blast cells are also high, the worse the prognosis. Blast cells are seen on blood smear in the majority of cases (blast cells are precursors -- stem cells -- to all immune cell lines). A bone marrow biopsy provides conclusive proof of ALL. A lumbar puncture (also known as a spinal tap) will indicate whether the spinal column and brain have been invaded.
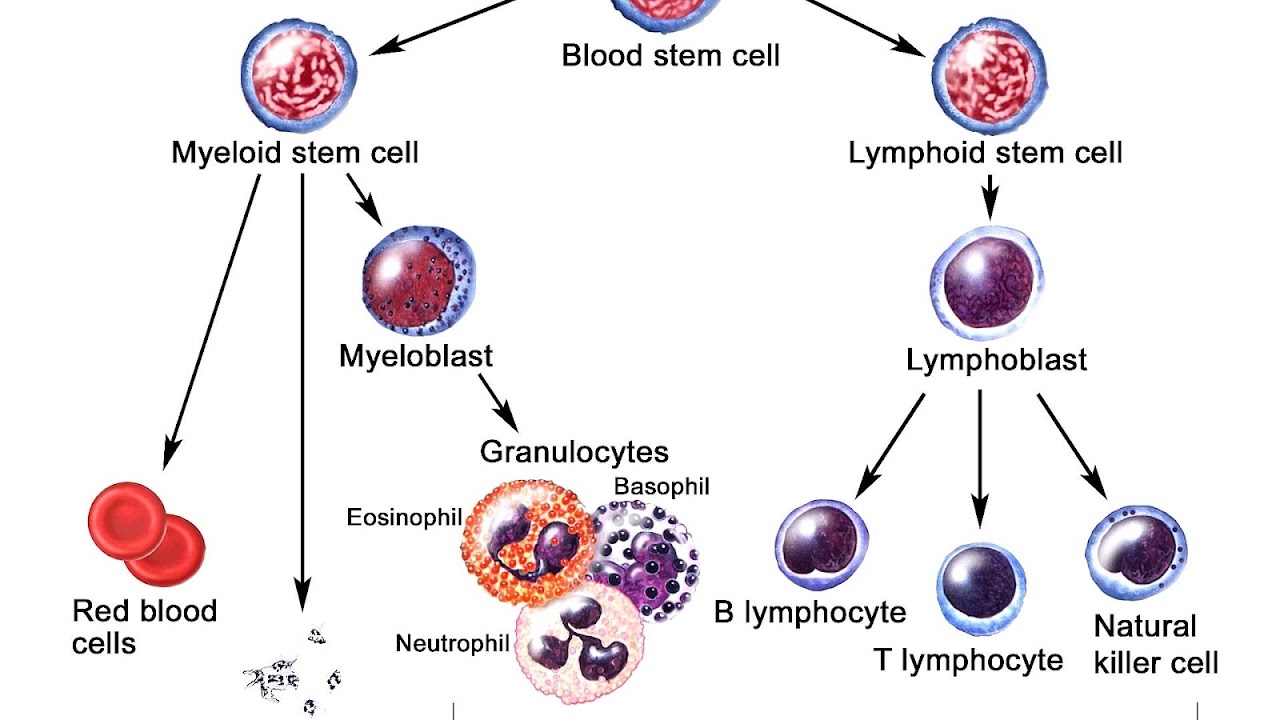
Pathological examination, cytogenetics (in particular the presence of Philadelphia chromosome), and immunophenotyping establish whether myeloblastic (neutrophils, eosinophils, or basophils) or lymphoblastic (B lymphocytes or T lymphocytes) cells are the problem. RNA testing can establish how aggressive the disease is; different mutations have been associated with shorter or longer survival. Immunohistochemical testing may reveal TdT or CALLA antigens on the surface of leukemic cells. TdT is a protein expressed early in the development of pre-T and pre-B cells, whereas CALLA is an antigen found in 80% of ALL cases and also in the "blast crisis" of CML.
Medical imaging (such as ultrasound or CT scanning) can find invasion of other organs commonly the lung, liver, spleen, lymph nodes, brain, kidneys, and reproductive organs.
Cytogenetics
Cytogenetic translocations associated with specific molecular genetic abnormalities in ALL
12;21 is the most common translocation and portends a good prognosis. 4;11 is the most common in children under 12 months and portends a poor prognosis.
Classification
As ALL is not a solid tumor, the TNM notation, used in solid cancers, has no relevance. Lymphoblastic lymphoma is a rare type of non-Hodgkin lymphoma, a result of abnormal adaptive immune cells, typically T-cells. It usually occurs in children.
Prior to 2008, subtyping of all acute leukemias (including acute myelogenous leukemia, AML) used the French-American-British (FAB) classification, in which ALL was classified as:
- ALL-L1: small uniform cells
- ALL-L2: large varied cells
- ALL-L3: large varied cells with vacuoles (bubble-like features).
The FAB scheme had only a limited impact on treatment choice. In 2008, the World Health Organization scheme identified three therapeutically distinct categories. These are identified by immunophenotyping of surface markers of the abnormal lymphocytes:
- B-lymphoblastic ALL (this category can be subdivided according to the correlation of the ALL cell immunophenotype with the stages of normal B-cell development)
- Burkitt ALL (corresponds to ALL-L3)
- T-cell ALL.
This subtyping helps determine the prognosis and the most appropriate treatment in treating ALL. It is substantially amplified by cytogenetics and molecular diagnostics tests.
Variant features
- Acute lymphoblastic leukemia with cytoplasmic granules
- Aplastic presentation of ALL
- Acute lymphoblastic leukemia with eosinophilia
- Relapse of lymphoblastic leukemia
- Secondary ALL
Immunophenotyping
The use of a TdT assay and a panel of monoclonal antibodies (MoAbs) to T cell and B cell associated antigens will identify almost all cases of ALL.
Immunophenotypic categories of acute lymphoblastic leukemia (ALL):
Treatment
The earlier ALL is detected, the more effective the treatment. The aim is to induce a lasting remission, defined as the absence of detectable cancer cells in the body (usually less than 5% blast cells in the bone marrow).
Treatment for acute leukemia can include chemotherapy, steroids, radiation therapy, intensive combined treatments (including bone marrow or stem cell transplants), and growth factors.
Chemotherapy
Chemotherapy is the initial treatment of choice. Most ALL patients will receive a combination of medications. There are no surgical options because of the body-wide distribution of the malignant cells. In general, cytotoxic chemotherapy for ALL combines multiple antileukemic drugs in various combinations. Chemotherapy for ALL consists of three phases: remission induction, intensification, and maintenance therapy.
As the chemotherapy regimens can be intensive and protracted (often about 2 years in case of the GMALL UKALL, HyperCVAD, or CALGB protocols; for ALL, about 3 years, 2 months for males on COG protocols, and 2 years, 2 months for females -- longer for males because the testicles are a potential reservoir), many patients have an intravenous catheter inserted into a large vein (termed a central venous catheter or a Hickman line), or a Portacath, a cone-shaped port with a silicone nose that is surgically planted under the skin, usually near the collar bone, and the most effective product available, due to low infection risks and the long-term viability of a portacath.
Radiation therapy
Radiation therapy (or radiotherapy) is used on painful bony areas, in high disease burdens, or as part of the preparations for a bone marrow transplant (total body irradiation). Radiation in the form of whole-brain radiation is also used for central nervous system prophylaxis, to prevent recurrence of leukemia in the brain. Whole-brain prophylaxis radiation used to be a common method in treatment of children's ALL. Recent studies showed that CNS chemotherapy provided results as favorable but with less developmental side-effects. As a result, the use of whole-brain radiation has been more limited. Most specialists in adult leukemia have abandoned the use of radiation therapy for CNS prophylaxis, instead using intrathecal chemotherapy.
Biological therapy
For some subtypes of relapsed ALL, aiming at biological targets such as the proteasome, in combination with chemotherapy, has given promising results in clinical trials. Selection of biological targets on the basis of their combinatorial effects on the leukemic lymphoblasts can lead to clinical trials for improvement in the effects of ALL treatment.
In ongoing clinical trials, a CD19-CD3 bi-specific monoclonal murine antibody, blinatumomab, is showing great promise.
Immunotherapy
Chimeric antigen receptors (CARs) have been developed as a promising immunotherapy for ALL. This technology uses a single chain variable fragment (scFv) designed to recognize the cell surface marker CD19 as a method of treating ALL.
CD19 is a molecule found on all B-cells and can be used as a means of distinguishing the potentially malignant B-cell population. In this therapy, mice are immunized with the CD19 antigen and produce anti-CD19 antibodies. Hybridomas developed from mouse spleen cells fused to a myeloma cell line can be developed as a source for the cDNA encoding the CD19 specific antibody. The cDNA is sequenced and the sequence encoding the variable heavy and variable light chains of these antibodies are cloned together using a small peptide linker. This resulting sequence encodes the scFv. This can be cloned into a transgene, encoding what will become the endodomain of the CAR. Varying arrangements of subunits serve as the endodomain, but they generally consist of the hinge region that attaches to the scFv, a transmembrane region, the intracellular region of a costimulatory molecule such as CD28, and the intracellular domain of CD3-zeta containing ITAM repeats. Other sequences frequently included are: 4-1bb and OX40. The final transgene sequence, containing the scFv and endodomain sequences is then inserted into immune effector cells that are obtained from the patient and expanded in vitro. In trials these have been a type of T-cell capable of cytotoxicity.
Inserting the DNA into the effector cell can be accomplished by several methods. Most commonly, this is done using a lentivirus that encodes the transgene. Pseudotyped, self-inactivating lentiviruses are an effective method for the stable insertion of a desired transgene into the target cell. Other methods include electroporation and transfection, but these are limited in their efficacy as transgene expression diminishes over time.
The gene-modified effector cells are then transplanted back into the patient. Typically this process is done in conjunction with a conditioning regimen such as cyclophosphamide, which has been shown to potentiate the effects of infused T-cells. This effect has been attributed to making an immunologic space within which the cells populate. The process as a whole results in an effector cell, typically a T-cell, that can recognize a tumor cell antigen in a manner that is independent of the major histocompatibility complex and which can initiate a cytotoxic response.
In 2017, a US Food and Drug Administration (FDA) advisory panel voted its unanimous positive recommendation of tisagenlecleucel as a CAR-T therapy for acute B-cell lymphoblastic leukaemia patients who did not respond adequately to other treatments or have relapsed. The FDA may or may not follow this recommendation. In a 22-day process, the "drug" is customized for each patient. T cells cells purified from each patient are modified by a virus that inserts genes that encode a chimaeric antigen receptor into their DNA, one that recognizes leukaemia cells.
Pregnancy
Leukemia is rarely associated with pregnancy, affecting only about 1 in 10,000 pregnant women. How it is handled depends primarily on the type of leukemia. Acute leukemias normally require prompt, aggressive treatment, despite significant risks of pregnancy loss and birth defects, especially if chemotherapy is given during the developmentally sensitive first trimester.
It is possible, although extremely rare, for leukemia to spread from the mother to the child. This is called vertical transmission.
Gene therapy
Tisagenlecleucel is a type of gene therapy used for B-cell acute lymphoblastic leukemia that has failed usual treatment. In August 2017, it became the first FDA-approved gene therapy in the United States. The medication requires customization to the person.

Prognosis
The five-year survival rate for children who have ALL has improved from zero, six decades ago, to 85% currently, largely because of clinical trials on new chemotherapeutic agents and improvements in stem cell transplantation (SCT) technology.
Five-year survival rates evaluate older, not current, treatments. New drugs, and matching treatment to the genetic characteristics of the blast cells, may improve those rates. The prognosis for ALL differs among individuals depending on a variety of factors:
- Gender: Females tend to fare better than males.
- Ethnicity: Caucasians are more likely to develop acute leukemia than African-Americans, Asians, or Hispanics. However, they also tend to have a better prognosis than non-Caucasians.
- Age at diagnosis: children 1-10 years of age are most likely to develop ALL and to be cured of it. Cases in older patients are more likely to result from chromosomal abnormalities (e.g., the Philadelphia chromosome) that make treatment more difficult and prognoses poorer.
- White blood cell count at diagnosis of less than 50,000/µl
- Cancer spread into the Central nervous system (brain or spinal cord) has worse outcomes.
- Morphological, immunological, and genetic subtypes
- Patient's response to initial treatment
- Genetic disorders such as Down syndrome
Cytogenetics, the study of characteristic large changes in the chromosomes of cancer cells, is an important predictor of outcome.
Some cytogenetic subtypes have a worse prognosis than others. These include:
- A translocation between chromosomes 9 and 22, known as the Philadelphia chromosome, occurs in about 20% of adult and 5% in pediatric cases of ALL.
- A translocation between chromosomes 4 and 11 occurs in about 4% of cases and is most common in infants under 12 months.
- Not all translocations of chromosomes carry a poorer prognosis. Some translocations are relatively favorable. For example, hyperdiploidy (>50 chromosomes) is a good prognostic factor.
- Genome-wide copy number changes can be assessed by conventional cytogenetics or virtual karyotyping. SNP array virtual karyotyping can detect copy number changes and LOH status, while arrayCGH can detect only copy number changes. Copy neutral LOH (acquired uniparental disomy) has been reported at key loci in ALL, such as CDKN2A gene, which have prognostic significance. SNP arrayvirtual karyotyping can readily detect copy neutral LOH. Array CGH, FISH, and conventional cytogenetics cannot detect copy neutral LOH.
Unclassified ALL is considered to have an intermediate prognosis.
Epidemiology
Acute lymphoblastic leukemia is seen in both children and adults; the highest incidence is seen between ages two and five years. ALL is the most common childhood cancer constituting about 23 to 30% of cancers before age 15. Although 80 to 90% of children will have a durable complete response with treatment it is the leading cause of cancer-related deaths among children. In adults, ALL is less common than acute myelogenous leukemia (AML). However, as there are more adults than children, the number of cases of ALL seen in adults is comparable to that seen in children. Its incidence has been estimated to be 1 in 1500 children.
Internationally, ALL is more common in children with Caucasian descent, being more common in Hispanics and in Latin America than in Africa.
United States
In the United States, the annual incidence of ALL is roughly 6,000 3,000-3,500, or approximately one in 50,000. ALL is slightly more common in males than females. In the United States in 2010, incidence from birth to age 19 was 38.4 per 1,000,000 per year in boys and 30.2 per 1,000,000 per year in girls. Prevalence was 30,171, and observed survival was 90% (based on data from 2003-2009). ALL has a bimodal age distribution, having a high incidence in ages 2-5 and another peak in incidence above 50 years old.
United Kingdom
ALL accounts for 8% of all leukemia cases in the United Kingdom; around 650 people were diagnosed with the disease in 2011.
Source of the article : Wikipedia

EmoticonEmoticon